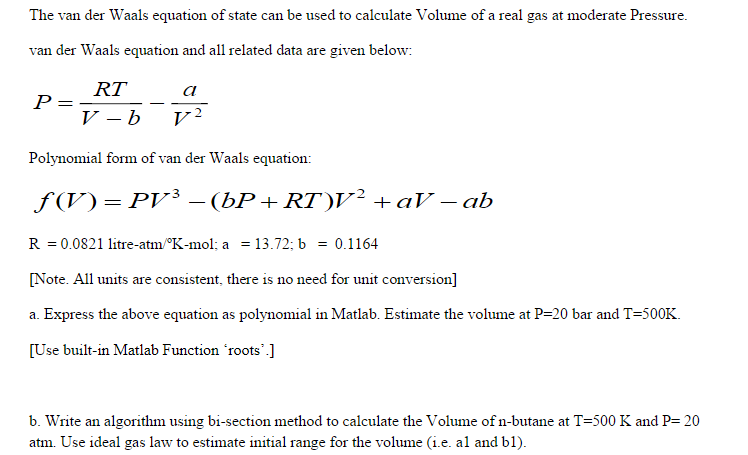
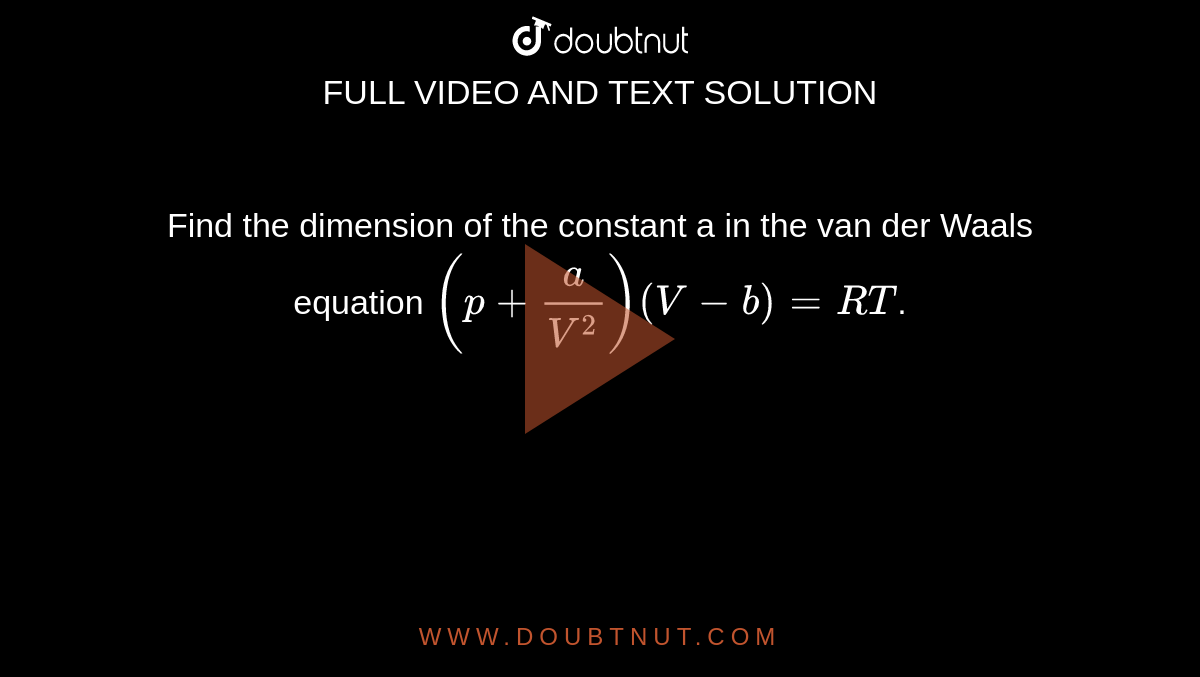
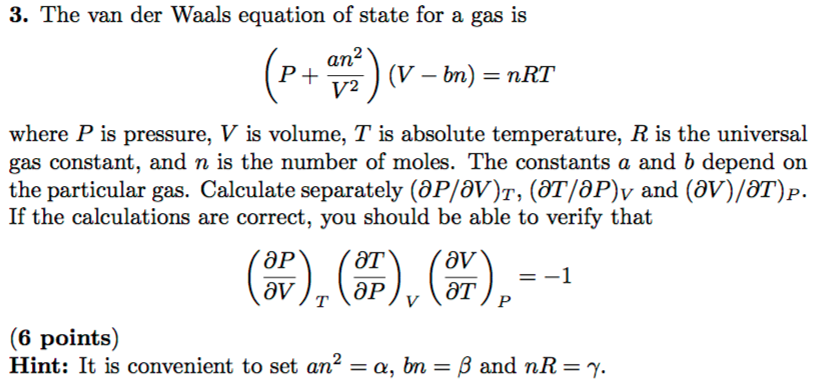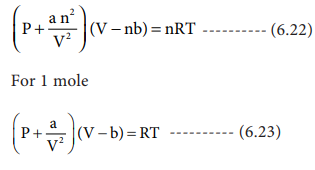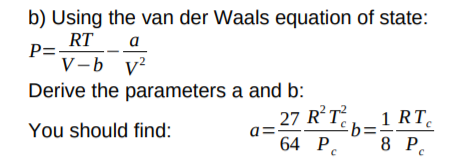= RT , at high pressure, the Van der Waals equation gets reduced to : Using Van der Waals equation, [ P + a/V^2 ](V - b) = RT , at high pressure, the Van der Waals equation gets reduced to :](https://dwes9vv9u0550.cloudfront.net/images/9876945/d6a3d03b-a449-4d51-986d-b05943344e70.jpg)
Using Van der Waals equation, [ P + a/V^2 ](V - b) = RT , at high pressure, the Van der Waals equation gets reduced to :
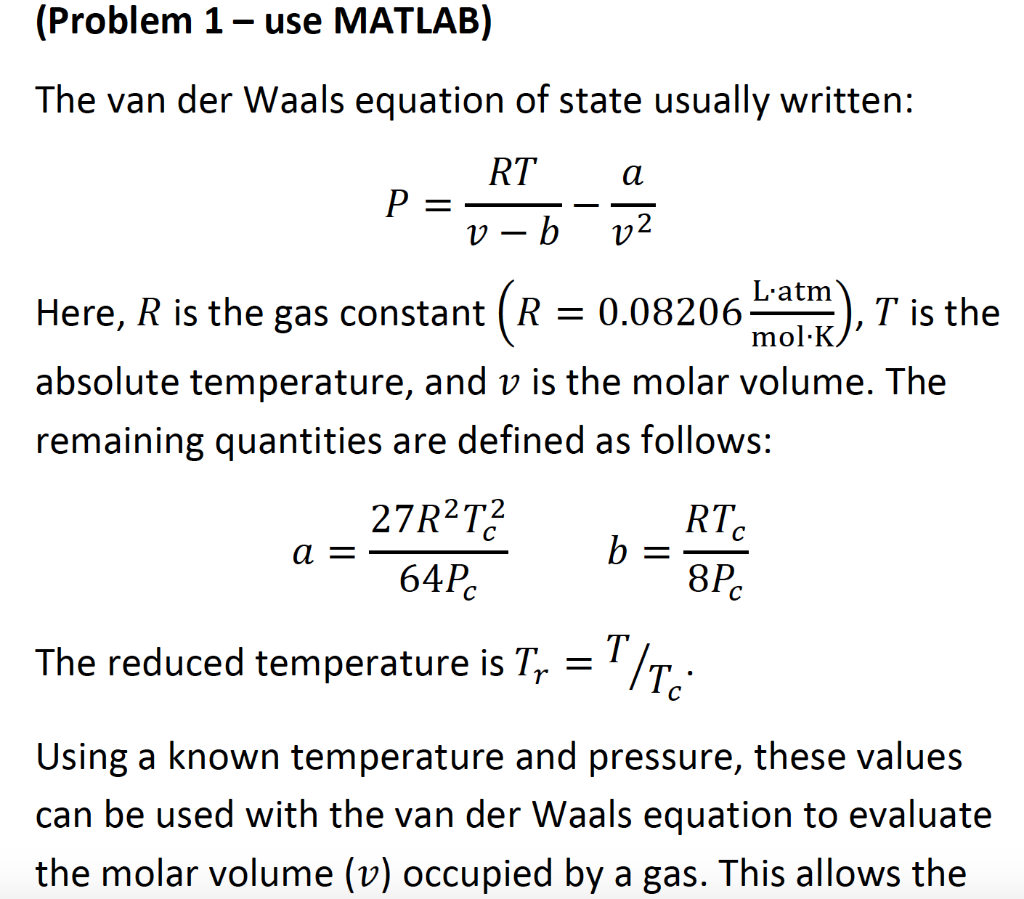
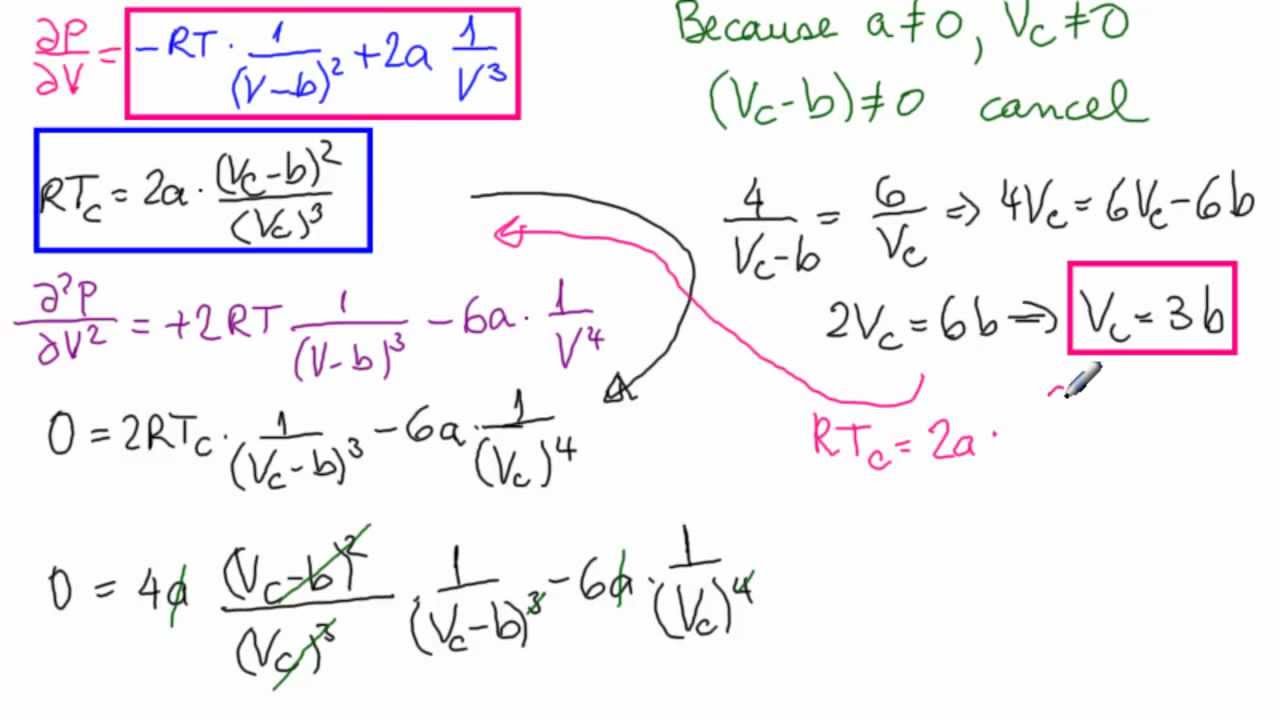
![SOLVED: Derive the equation: dU = [6)v P]av + Cv dT Based on this, calculate for the van der Waals gas with the equation of state (a b are constants): (p+a6) )v - SOLVED: Derive the equation: dU = [6)v P]av + Cv dT Based on this, calculate for the van der Waals gas with the equation of state (a b are constants): (p+a6) )v -](https://cdn.numerade.com/ask_images/8b2fa2c641c442aab9bd82238063f386.jpg)
SOLVED: Derive the equation: dU = [6)v P]av + Cv dT Based on this, calculate for the van der Waals gas with the equation of state (a b are constants): (p+a6) )v -
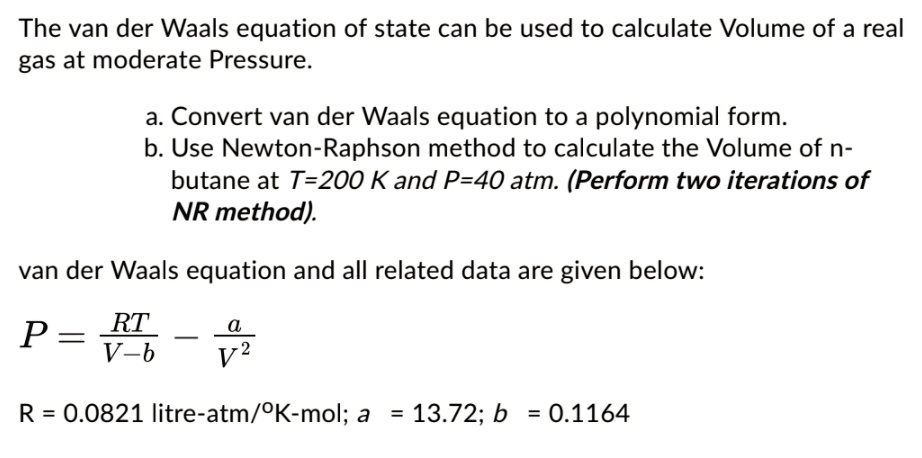
SOLVED: The van der Waals equation of state can be used to calculate Volume of a real gas at moderate Pressure: a. Convert van der Waals equation to polynomial form b. Use

Thermodynamics - Work done calculation for van der Waals gases under isothermal conditions. - YouTube
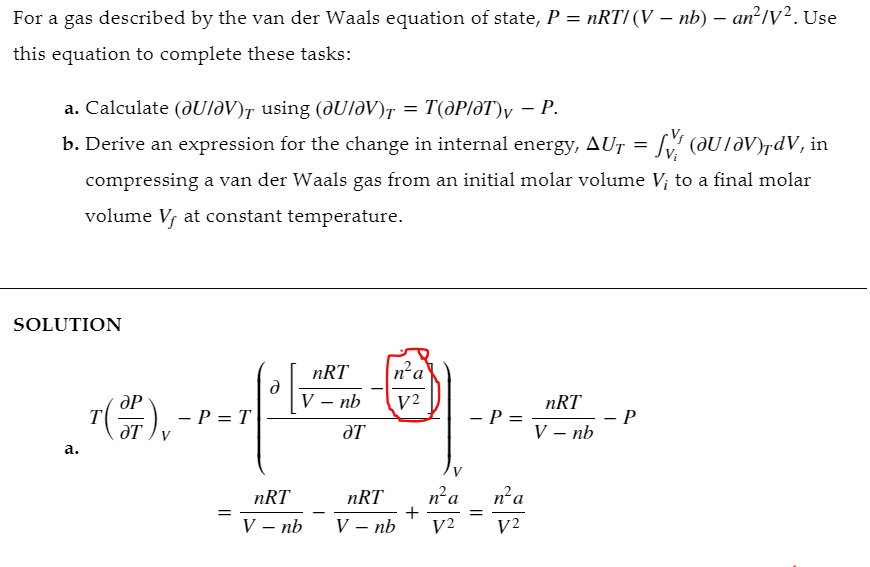
SOLVED: For a gas described by the van der Waals equation of state, P = nRTI (V nb) an?/V? . Use this equation to complete these tasks: Calculate (JUIdV)r using (JUIIV)T T(PIdT)v -