If Avogadro number `N_(A)` is changed from `6.022xx10^(23) mol^(-1)` to 6`.022xx10^(23) mol^(-1)`, - YouTube
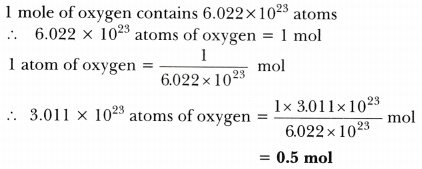
Calculate the number of moles present in: 3.011 X 10^23 number of oxygen atoms. 60 g of calcium - CBSE Class 9 Science - Learn CBSE Forum

Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

If Avogadro number NA is changed from 6.022 × 10^23 mol^-1 to 6.022 × 10^20 mol^-1 , this would change :

1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com

One mole of any substance contains 6.022xx10^(23) atoms/molecules. Number of molecules of H(2)SO(4) present in 100mL of 0.02M H2SO(4) solution is

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
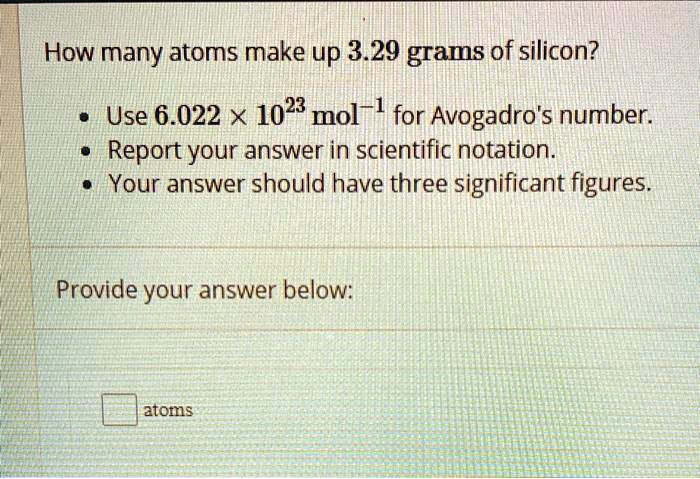
SOLVED: How many atoms make up 3.29 grams of silicon? Use 6.022 X 1023 mol-l for Avogadro's number: Report your answer in scientific notation. Your answer should have three significant figures Provide
Summary Examples Page 1 of 2 Definitions of a mole 1/24/2007 http://www.ausetute.com.au/moledefs.html